Grass Pollen Surface Ornamentation a Review of Morphotypes and Taxonomic Utility
Introduction
Pollen grains of the family Poaceae are widely found in Quaternary sediments of southern Brazil (e.g., Behling et al., 2004; Macedo et al., 2007; Bauermann et al., 2008). Notwithstanding, the stenopalynous nature of the pollen from this family makes it hard to determine subfamilies and genera using pollen data (Erdtman, 1952; Salgado-Labouriau, 1973). Consequently, depression taxonomic resolution hampers paleoecological inferences. Being mainly associated with grassland vegetation (Figure 1), Poaceae pollen is usually interpreted as indicative of open up formations. However in Rio Grande do Sul (RS), where more than 80% of Poaceae species occupy grasslands, a significant pct (twenty%) of representatives of this family inhabit forest vegetation (Boldrini and Longhi-Wagner, 2011).

Effigy 1. (A) Grassland vegetation of Rio Grande do Sul, "Cerro exercise Ouro," São Gabriel urban center. (B) Grassland of the coastal plain of RS, "Balneário Quintão," Palmares do Sul city. (C,D) Riparian forest with lignified bamboos, Gravataí city.
Although the Poaceae family is well represented and studied in RS, few studies accept examined the pollen representatives of this family. The few descriptions or illustrations of pollen representatives presented in the study by Tedesco et al. (1999), which analyzed the bore of the pollen grains of Hemarthria altissima under different ploidy levels, noted that the boilerplate diameters were variable, depending on the ploidy. Nonetheless, the analyzed pollen grains were not acetolyzed. Medeanic et al. (2008) illustrated images of pollen grains from nine species, while Wilberger et al. (2004) presented images of pollen grains corresponding to 3 separate species. Nakamura et al. (2010), addressing the evolution of anther and pollen grains in Axonopus aureus, Chloris elata, h Eragrostis solida, Olyra humilis, Paspalum polyphyllum, and Sucrea monophylla, found similar pollen morphology betwixt taxa. This excludes those grains that have been observed to have patterns that are important for differentiation betwixt species of the family. Radaeski et al. (2011) described the pollen morphology of Paspalum notatum, Paspalum plicatulum, and Schizachyrium microstachyum. After, Bauermann et al. (2013) described the pollen grains of Andropogon lateralis and Eragrostis bahiensis. Radaeski et al. (2014a) described the pollen morphology of Eragrostis neesii, and Radaeski et al. (2014b) contributed to the pollen description of half-dozen taxa of Poaceae, which showed that, in general, the pollen grains are of average size, with a monoaperture and spherical forms, and noted the identified stenopalynous characteristic of the family unit.
In Due south America, studies take been conducted on Poaceae pollen grains from Venezuela, Chile, Brazil, and Argentina (Heusser, 1971; Markgraf and D'Antoni, 1978; Salgado-Labouriau and Rinaldi, 1990). In Republic of chile, the overlap between dimensions of the pollen grains of some tribes made it hard to differentiate between tribes or subfamilies (Heusser, 1971). Thus, further studies on the pollen grains from other taxa are needed. Besides, the same is true of the pollen grains of Barro Colorado Island (Roubik and Moreno, 1991). Withal, analyses of many species that are based solely on one pollen grain cannot verify the variations in total pollen grain diameter of the species. Analyzing pollen grains from Argentina, Markgraf and D'Antoni (1978) observed—from other grassland species studied—the largest diameter of pollen grain of the bamboo Chusquea culeou. Thus, analysis of pollen grains from other wood species can provide patterns related to vegetation from this site.
The pollen grains of many species from other regions of the world (due east.1000., Europe, Southward America) accept been analyzed past scanning electron microscopy (SEM). Some of these species also inhabit southern Brazil. Some studies (Table 1) take explored the surface of the Poaceae pollen grains through SEM, which has contributed to separation of taxonomic groups (Köhler and Lange, 1979; Linder and Ferguson, 1985; Chaturvedi et al., 1994, 1998; Chaturvedi and Datta, 2001; Skvarla et al., 2003; Datta and Chaturvedi, 2004; Liu et al., 2004, 2005; Perveen, 2006; Kashikar and Kalkar, 2010; Ahmad et al., 2011; Dórea, 2011; Perveen and Qaiser, 2012; Mander et al., 2013, 2014; Nazir et al., 2013; Morgado et al., 2015; Needham et al., 2015; Mander and Punyasena, 2016). However, other studies using light microscopy (LM) have shown morphometric differences in the size of the Poaceae pollen grain species (Salgado-Labouriau and Rinaldi, 1990; Katsiotis and Forsberg, 1995; Joly et al., 2007; Schüler and Behling, 2011a,b; Jan et al., 2014). In add-on, when analyzing large sets of palynomorphs from 4th sediments, the use of SEM is a difficult and time-consuming job. Thus, morphometric datasets may be valuable for utilise in studies of fossil Poaceae pollen analysis (Schüler and Behling, 2011a,b; Jan et al., 2014).
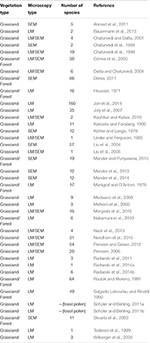
Table 1. Dataset of Poaceae analyzed species, type of microscopy used and vegetation type.
Recently, studying pollen grains of fossil Poaceae in the grassland ecosystems of South America, Schüler and Behling (2011a) discovered potential new ways to distinguish grassland types. In their later study, Schüler and Behling (2011b) were able to differentiate the ecosystems present in Due south America. Moreover, Jan et al. (2014) succeeded in identifying a blueprint amongst changes in the size of Poaceae pollen grains co-ordinate to the ploidy level and C3 and C4 metabolism, thereby demonstrating that polyploid species accept a larger pollen grain size. C4 species are tropical and inhabit warmer and drier regions, while temperate Poaceae species are C3 and alive in humid and cold conditions (Boldrini, 2006). C3 and C4 species are important for paleoclimate studies because they indicate past variation in temperature and precipitation (Schüler and Behling, 2011a).
The aim of the study was to distinguish Poaceae pollen grains from grassland and forest vegetation of southern Brazil. The pollen grains of 68 species were analyzed to answer the following questions: (i) Tin can Poaceae pollen grains be separated into those of grassland species and those of forest species? (two) Practise the pollen grains of forest species differ in size co-ordinate to their arboreal or herbaceous habit?
Materials and Methods
Collection of Botanical Cloth
During the field expeditions, 98 specimens of Poaceae were obtained, anticipating the 21 taxa representatives of this family, and some pollen material, being fertile, was selected for extraction. To obtain hibernal and estival plants in the flowering seasons, the samples were gathered using the transversal method (Filgueiras et al., 1994) in wintertime, fall, spring, and summertime in May, August, September, October, November, and Dec of 2013, besides every bit in January of 2014.
Later on collection, the plants were pressed and dehydrated. The plants were identified by a skilled taxonomist (A. A. Schneider). The drove of herbarium specimens was deposited in the "Herbário practice Museu de Ciências Naturais" from the Universidade Luterana do Brasil (MCNU/HERULBRA), and duplicates were deposited in the "Herbário Bruno Edgar Irgang" from Unipampa (HBEI/UNIPAMPA). The anthers were collected for chemical treatment of the herbarium materials from other Poaceae species. Since some species have state-restricted distribution, or sporadic bloom periods, samples were collected from pollen cloth in accordance with information provided by the ICN Herbarium (Effigy 2, Table 2).
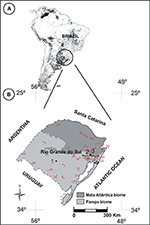
Effigy ii. (A) Distribution of grasslands in South America highlighting the southern region of Brazil (adapted from Eva et al., 2002). (B) Map of Rio Grande do Sul showing the sampling sites in RS (black circles): ane. "Cerro do Ouro," São Gabriel urban center; 2. Cachoeirinha city; 3. "Sitio Laranjal," Gravataí metropolis; 4. "Balneário Quintão," Palmares do Sul city. Ruby-red circles indicate regions of collection of herbarium species (see Tabular array ii for more details of the names of regions).

Table two. Information of the examined cloth in the Rio Grande practice Sul, Brazil.
The drove of pollen materials from a herbarium (containing plants from different regions of the country) enabled the authors of this written report to institute an overview of the modern grass pollen of RS. Past combining the findings of this study with the work of Hasenack et al. (2010) on the vegetation physiognomy of the land, we were able to found a human relationship between the pollen and the regional flora. Only 50 Poaceae species of four subfamilies comprise forest vegetation. Thus, we considered the selection of 10 woods species from all tribes and subfamilies for pollen analysis to be adequate. More than species of grassland (58 species) were analyzed because there are a greater number of Poaceae species (450 species) in southern Brazil (Boldrini and Longhi-Wagner, 2011) than forest species.
Handling and Clarification of Pollen Grains
The anthers were chemically processed according to the acetolysis methodology proposed by Erdtman (1952). Later acetolysis, using glycerinated jelly, 5 permanent slides were created for each sample and deposited in the Laboratório de Palinologia da ULBRA. The pollen grains were measured and the slides mounted on the same day to prevent any changes in pollen size (Salgado-Labouriau, 2007). Schüler and Behling (2011a) measured 60 pollen grains from fossil pollen samples, but in this paper we studied modern pollen grains, measuring 25 pollen grains according to the methodology used for the report of modernistic pollen grains (Erdtman, 1952; Barth and Melhem, 1988).
The morphological characteristics of the pollen grains were observed and described by LM. A Leica CME microscope was used for measurements and for recording images. Using a × grand magnification, nosotros recorded the polar diameter (P), equatorial bore (East), or only the diameter (D) of spherical pollen grains, and the thickness of the exine (Ex) in 25 randomly selected pollen grains. In addition to the above, measurements of the pore and annulus width of the studied species were recorded (Figure 3). The pollen grains were so described in regard to their pollen unit, size, symmetry, polarity, amb, type of aperture, and decoration, using the terminology proposed by Barth and Melhem (1988) and Punt et al. (2007).
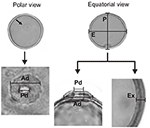
Figure 3. The parameters of a Poaceae pollen grain are considered in the present paper. Polar diameter (P), equatorial diameter (Due east), pore diameter (Pd), annulus diameter (Ad), and exine thickness (Ex).
Statistical Analysis
BioEstat 5.0 and PAST 3.05 software was used for the statistical analysis. BioEstat 5.0 software was used to compile a frequency distribution histogram of pollen grain sizes. The histogram was then constructed from the size of the pollen grains of species inhabiting arboreous wood, grassland, and herbaceous forest. This program (BioEstat v.0) was too used to determine size differences among pollen grains from arboreous forest, grassland, and herbaceous forest species using One-mode ANOVA followed by Tukey's exam. Past 3.05software was used for discriminant assay (DA) of dissever groups according to the size of the pollen grains. In DA nosotros utilise the minimum, maximum, and boilerplate size of pollen grains of all species to determine whether they tin be grouped. We used this program (PAST iii.05) as well to show the correlation between pollen grain, annulus, and pore sizes by Pearson correlation. The Pearson'southward correlation coefficient examination was used to determine the boilerplate size of the pollen grains, the pore width, and the annulus width for all species (68 species studied). The PAST 3.05 software was also used to construct a box plot. The box plot shows overlapping measures and measures that practice not overlap. We used all the pollen grain size measurements for the box plot (i.e., 25 measures each of 68 species = full 1700 measures).
Results
Measurement of Pollen Grains
Table 3 presents the measurements of the pollen grains for the 68 species, in evolutionary order according to the GPWG (Grass Phylogeny Working Group) classification (2001). Still, differences were observed in the measurements of pollen grains, pores, and annulus. Pollen grains of species of each tribe were selected to testify the Poaceae morphological characteristics of all the tribes of southern Brazil (Figures 4–eight).
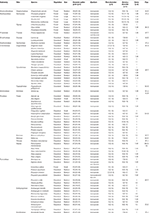
Table iii. Pollen morphological measurements of 68 Poaceae species in the region of southern Brazil.

Figure 4. Pollen grains of the subfamilies Anomochlooideae, Bambusoideae, and Pharoideae. (A–E) Streptochaeta spicata: PV (A), EV (B), detail of ornamentation (C), detail of the thickness of the exine (D), and detail of the aperture (East); (F–J) Chusquea juergensii: PV (F), EV (G), detail of ornamentation (H), detail of the thickness of the exine (I), and detail of the discontinuity (J); (1000–O) Olyra latifolia: PV (K), EV (L), particular of ornamentation (Chiliad), detail of the thickness of the exine (North), and detail of the discontinuity (O); (P–T) Pharus lappulaceus: PV (P), EV (Q), detail of ornamentation (R), detail of the thickness of the exine (South), and item of the aperture (T).

Effigy 5. Pollen grains of the subfamilies Ehrarthoideae, Danthonioideae, and Chloridoideae. (A–Eastward) Leersia sp.: PV (A), EV (B), particular of decoration (C), detail of the thickness of the exine (D), and detail of the discontinuity (E); (F–J) Danthonia montana: PV (F), EV (1000), detail of ornament (H), detail of the thickness of the exine (I), and detail of the aperture (J); (K–O) Eragrostis neesii: PV (K), EV (50), detail of ornamentation (Chiliad), detail of the thickness of the exine (N), and detail of the aperture (O); (P–T) Chloris canterae: PV (P), EV (Q), particular of ornamentation (R), detail of the thickness of the exine (S), and detail of the aperture (T).

Figure half dozen. Pollen grains of the subfamilies Chloridoideae, Aristidoideae, and Pooideae. (A–E) Pappophorum philippianum: PV (A), EV (B), item of decoration (C), detail of the thickness of the exine (D), and detail of the discontinuity (E); (F–J) Aristida sp.: PV (F), EV (One thousand), particular of ornamentation (H), item of the thickness of the exine (I), and detail of the aperture (J); (Grand–O) Chascolytrum subaristatum: PV (K), EV (L), detail of ornamentation (One thousand), item of the thickness of the exine (Northward), and particular of the discontinuity (O); (P–T) Bromus catharticus: PV (P), EV (Q), item of ornamentation (R), detail of the thickness of the exine (South), and detail of the aperture (T).

Figure 7. Pollen grains of the subfamilies Pooideae and Panicoideae. (A–Eastward) Melica sp.: PV (A), EV (B), item of ornamentation (C), detail of the thickness of the exine (D), and detail of the discontinuity (Due east); (F–J) Hordeum stenostachys: PV (F), EV (G), detail of decoration (H), detail of the thickness of the exine (I), and item of the aperture (J); (K–O) Piptochaetium montevidense: PV (K), EV (50), particular of ornament (M), particular of the thickness of the exine (North), and detail of the discontinuity (O); (P–T) Paspalum urvillei: PV (P), EV (Q), detail of ornamentation (R), detail of the thickness of the exine (S), and detail of the discontinuity (T).

Figure 8. Pollen grains of the subfamily Panicoideae. (A–E) Schizachyrium microstachyum: PV (A), EV (B), detail of ornamentation (C), item of the thickness of the exine (D), and particular of the discontinuity (E); (F–J) Arundinella hispida: PV (F), EV (One thousand), detail of ornamentation (H), detail of the thickness of the exine (I), and detail of the aperture (J).
A frequency distribution histogram of the measurements of pollen size is shown in Figure 9A. The boilerplate measurement values were college for arboreous woods (eight% of the measurements of these species were l μm). Grassland and herbaceous forest species had lower average measurement values (sixteen% of the forest herbaceous species measured 27 μm, and 8% of the grassland species measured 32 μm). Grain size distributions showed a Gaussian distribution for samples of arboreous forest, grassland, and herbaceous forest species (Figures 9B–Eastward). The Gaussian distribution showed that ANOVA-Tukey tin be applied to the data set.
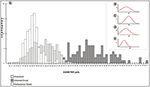
Effigy 9. Frequency distribution histogram of the pollen size measurements (A). Gaussian distribution of size measurements of pollen grains of arboreous species (B), grassland species (C), herbaceous woods species (D), and all species studied (Due east). F, frequency; S, pollen grain size.
Morphometric Variation in Diameters of the Pollen Grains, Pores, and Annulus
The ANOVA-Tukey test showed statistically meaning differences between the size of pollen grains of arboreous woods, grassland, and herbaceous forest species (Table iv). This divergence is clear in the comparing of samples that point values (p) less than 0.01. The difference between the means of the arboreous forest and grassland samples was large (22.1719), while among the grassland and herbaceous forest samples the deviation was pocket-sized (4.3681).
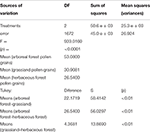
Table 4. Significances between the size of pollen grains of forest arboreous, grassland, and wood herbaceous species obtained with ANOVA-Tukey.
The DA exam (Effigy ten) determined the separation of three groups according to the values of the variables. The DA identified the separation of arboreous forest grassland and herbaceous wood groups. The arboreous forest group showed the greatest differences, and the grassland and herbaceous forest groups were mixed.

Figure 10. Discriminant analysis of the pollen grain size ordination of the 68 Poaceae species.
The Pearson correlation test (Table v) showed values that betoken a strong human relationship between the size of pollen grains and the width of the pore (r = 0.8281). It also showed a strong human relationship between the size of pollen grains and the width of the annulus (r = 0.8565). The diagrams of the values obtained indicate a similarity between the size of pollen grains, the pore width, and the width of the annulus (Effigy 11).

Tabular array five. Pearson correlation coefficient values showing the strength of relationship among the pore, annulus and size of the pollen grain.
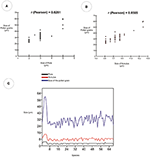
Figure 11. Pearson correlation diagram showing the force of the human relationship between pore, annulus, and size of pollen grain for the 68 Poaceae species. (A) Correlation between the variable sizes of the pore and size of the pollen grain. (B) Correlation between the variable annulus sizes and the pollen grain sizes. (C) Linear chart of the values of the diameters of the pores, annulus, and pollen grains.
All taxa showed monoporate apertures with annulus effectually the pores, except for Pharus lappulaceus, Digitaria ciliares, and Paspalum pauciciliatum, which showed diporate as well as the monoporate pollen grains. However, these three species (P. lappulaceus, D.ciliares, and P. pauciciliatum) showed just a few diporate pollen grains; nearly of their pollen grains were institute to exist monoporate. Still, they were unique species in terms of having diporate pollen. In the herbaceous forest species with diporate pollen grains, the grains measured 23–27 μm in width, while in the grassland species with diporate pollen, the grains measured 34–46 μm in width.
Interpretation of, and Distinction between, the Grassland and Forest Pollen Grains
In southern Brazil, 80% of Poaceae species are grassland species, while xx% are forest species. In our information set (68 species), 85.29% were grassland species and 14.71% were forest species. Thus, we analyzed the appropriate proportions of species relating to grassland and woods vegetation in the region.
The box plot of data sets relating to different Poaceae vegetation (arboreous forest, grassland, and herbaceous forest) showed pollen grains of different size ranges (Effigy 12). The pollen grains of arboreous woods species were larger than those of grassland and herbaceous forest species. The pollen grains of grassland species and herbaceous wood species were plant to be of similar size. However, the pollen of the grassland species had a lower minimum size than that of the wood herbaceous species. Three pollen types could be separated based on pollen grain size (Table 6). The Bambuseae pollen blazon was establish to accept pollen grains larger than 46 μmin width. Pollen grains that vary in size between 22 and 46 μm are of the herbaceous pollen type; these pollen grains vest to either grassland or herbaceous forest species. The grassland pollen blazon has small-scale pollen grains, measuring less than 22 μm.
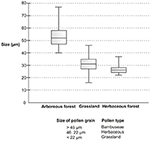
Figure 12. Nautical chart box plot of the diameters of the pollen grains. The bold horizontal line inside the box represents the median. The box shows 50% of the interquartile range, and whiskers the total variation.

Table 6. Pollen measures and establishment of pollen types.
Discussion
Based on measurements of pollen grains, previous studies take allowed scholars to distinguish between Poaceae pollen grains of South American ecosystems, and also to show the trends in pollen grain size among Ciii and C4 Poaceae species (Schüler and Behling, 2011a,b; Jan et al., 2014). In this work, information technology was possible to distinguish the Poaceae pollen grains relating to grassland and forest species of southern Brazil. January et al. (2014) analyzed a big data set with species from various locations effectually the world. In our work we wanted to analyze the variability within 1 ecosystem; therefore, nosotros chose to analyze a large set of data relating to but ane region (southern Brazil).
Studies of Poaceae pollen grains have revealed a strong correlation betwixt size of pollen grain, pore, and annulus (Skvarla et al., 2003; Joly et al., 2007; Schüler and Behling, 2011a,b; January et al., 2014). The results of our ain study too showed a relationship between size of pollen, pore, and annulus, as determined through correlation assay.
Analysis of the Poaceae pollen of the plants deposited in the herbarium provided a clarification of the variation in pollen grain size of species that occur in different regions of the state of RS. According to the results, relating pollen data to information on the current vegetation of RS, the main variations in size of Poaceae pollen grains in the state (Figure 13) could be mapped. Taking into business relationship the vegetation types that are based on more representative genera from different regions (Hasenack et al., 2010), nosotros can assign to regions the probable main pollen types occurring in different locations. Thus, the northern half of RS seems to be composed of larger pollen grains. We also found a reduction in size toward the southern half of the country, where the concentration of smaller pollen grains can be associated with the western part of RS, especially in the region of grassland with shallow soils (where the range of diameters for pollen grains is 22–34 μm).
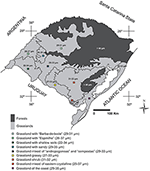
Figure 13. Chief changes in the average size of the Poaceae pollen grains in the unlike regions of Rio Grande do Sul, co-ordinate to the vegetation physiognomies in RS (adjusted from Hasenack et al., 2010).
Forest Vegetation
Pollen grains of wood Poaceae species showed distinctions between species. Arboreous species showed larger pollen grains than herbaceous species. The pollen grain size of the arboreous species ranged from medium to large, while that of the herbaceous species ranged from small-scale to medium. The pollen grains of arboreous Poaceae species showed a tendency toward larger sizes (Markgraf and D'Antoni, 1978; Salgado-Labouriau and Rinaldi, 1990). The differently sized grains of pollen woods species may be related to the pocket-sized wind flow inside the forests and may likewise be influenced past pollination (Dórea, 2011). Some variations in the size of the pollen grains of modernistic Poaceae species has already been reported in Due south America. In Venezuela, larger pollen grains take already been reported to exist related to the Bambusoideae and Pooideae subfamilies (Salgado-Labouriau and Rinaldi, 1990). However, in southern Brazil, nosotros are able to differentiate at the level of tribes determining the Bambuseae pollen blazon. The Bambuseae pollen blazon is indicative of arboreal grasses and humid regions (Schmidt and and Longhi-Wagner, 2009).
Grassland Vegetation
Pollen grains of grassland species are smaller than those of arboreous forest species and similar to those of herbaceous wood species. These results make information technology possible to place the herbaceous pollen type. The smaller size of pollen grains in grassland species allows identification of the grassland pollen type. The small and medium sizes of pollen grains of grassland Poaceae species correspond to previous data relating to South American species (Heusser, 1971; Markgraf and D'Antoni, 1978; Salgado-Labouriau and Rinaldi, 1990; Melhem et al., 2003; Côrrea et al., 2005; Bauermann et al., 2013; Radaeski et al., 2014a,b) and species from other regions of the world (Joly et al., 2007; Jan et al., 2014; Morgado et al., 2015). The small-scale size of pollen grains of grassland species can as well exist related to the type of dispersion involved, since grassland species produce more than pollen than forest species (Radaeski and Bauermann, 2016).
Exine
Many studies have revealed differences in the exine sculpture of Poaceae pollen grains. Such differences are evident through the apply of SEM, which allows adequate analysis of the surface (Köhler and Lange, 1979; Linder and Ferguson, 1985; Chaturvedi et al., 1994, 1998; Chaturvedi and Datta, 2001; Skvarla et al., 2003; Datta and Chaturvedi, 2004; Liu et al., 2004, 2005; Perveen, 2006; Kashikar and Kalkar, 2010; Ahmad et al., 2011; Dórea, 2011; Perveen and Qaiser, 2012; Mander et al., 2013, 2014; Nazir et al., 2013; Morgado et al., 2015; Needham et al., 2015; Mander and Punyasena, 2016). Calorie-free microscopy is used to study the fossil pollen of 4th sediments at smaller magnifications (× 400); SEM is not suitable for such study. Thus, data pollen measures seem to be more than suitable to use in comparison with fossil pollen.
The thin exine of the Poaceae pollen grains is a remarkable feature, not exceeding 2 μm in thickness and having equivalent sexine and nexine values. Because of this thin layer, many pollen grains—peculiarly the larger ones—may display small changes in their spherical shape owing to the flattening of the pollen grain. This oftentimes provides the impression of pollen grains with prolate or oblate forms. However, these shapes are hands observed in crushed pollen grains, for when the not-deformed (used equally parameters) pollen grains are examined, their spherical course—characteristic of the Poaceae family—is noted.
The surface of the exine of Poaceae pollen grains, when viewed past SEM, exhibits several variations among species (Dórea, 2011). Nonetheless, when observed under light microscopy, the pollen surface exhibits a tectate exine with columellae and spinulose ornament. The variations in the surface of the exine observed past SEM cannot be observed past light microscopy. To identify the pollen grains (from pollen records) involving smaller increases, mainly occurring in Fourth sediments, the decoration is frequently not observed. With a magnification of × 400, much ornamentation of the studied taxa is not visible (Schüler and Behling, 2011a,b), for the ornamentation is often interpreted every bit psilate, scabrate, or microrreticulate surfaces. However, under higher (SEM) magnifications, the sculptured grain surfaces may be evident (Chaturvedi et al., 1998; Liu et al., 2004; Dórea, 2011; Mander et al., 2013).
Conclusions
Using a data set of 68 species, we found that types of vegetation tin can be distinguished according to Poaceae pollen grains. The size of pollen grains of arboreous forest Poaceae species differs from that of grassland and herbaceous woods species. The pollen grains of woods species of arboreal addiction are larger than those of forest species of herbaceous habit. Through measurements and statistical assay, we found that these Poaceae species showroom variation in the size of pollen grains in species inhabiting arboreous woods, grassland, and herbaceous forest. Thus, three pollen types were identified: Bambuseae, herbaceous, and grassland pollen types.
Grassland and forest vegetation may be distinguished by examining Poaceae pollen grains from southern Brazil. Thus, the dynamics of the grassland and wood vegetation during the Pleistocene and Holocene periods tin exist demonstrated based on Poaceae pollen grains. Also, pollen characterization by vegetation is of great importance, since the pollen morphology of the grasslands and forests tin be used as indicators of humid or dry environments, respectively.
Past determining the size of pollen grains of 68 Poaceae species in RS, information technology was possible to point previously inaccessible data for ecological inferences concerning southern Brazil grasslands. Attaining meliorate taxonomic resolution of both vegetation types allows new opportunities to expand the pollen records beyond the family unit level. Farther research is needed on the pollen morphology of other native genera and species of the Poaceae family for RS. It is expected that farther studies will allow greater differentiation between groups and improved knowledge of pollen morphology at a family level. The presented method is being applied to the evolution of pollen records for southern Brazil and may favor climactic reconstruction of past environments and an evaluation of the dynamics of 4th grassland Poaceae vegetation.
Writer Contributions
JR provided the images of the pollen grains and pollen measurements. JR, SB, AP structured and edited the manuscript during all phases. SB and AP supervised the project. JR and SB supported the paleoecological interpretations. JR and AP developed the botanical implications.
Disharmonize of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential disharmonize of interest.
Acknowledgments
This work is part of the M.Sc. dissertation of the offset authors, sponsored past CAPES. Our appreciation goes to Dr. Angelo Alberto Schneider from UNIPAMPA, for the nifty help with the identification of botanical materials. Likewise, we desire to thank the ICN herbarium. Nosotros give thanks Jim Kernaghan and Guy Barcellos for the linguistic revision.
References
Ahmad, F. A., Khan, Chiliad. A., Ahmad, M., Zafar, One thousand., Khan, A., and Iqbal, Z. (2011). Palynological studies in tribe Chlorideae (Poaceae) from table salt range of Pakistan. Afr. J. Biotechnol. 10, 8909–8913. doi: x.5897/AJB10.2512
CrossRef Total Text | Google Scholar
Barth, O. Grand., and Melhem, T. S. (1988). Glossário Ilustrado de Palinologia. Campinas: Editora da UNICAMP.
Bauermann, S. G., Macedo, R. B., Behling, H., Pillar, 5., and Neves, P. C. P. (2008). Dinβmicas vegetacionais, climáticas e practise fogo com base em palinologia e análise multivariada no Quaternário Tardio no sul do Brasil. Rev. Bras. Paleontol. eleven, 87–96. doi: x.4072/rbp.2008.2.02
CrossRef Full Text
Bauermann, S. G., Radaeski, J. N., Evaldt, A. C. P., Queiroz, East. P., Mourelle, D., Prieto, A. R., et al. (2013). Pólen nas Angiospermas: Diversidade e Evolução. Canoas: Editora da ULBRA.
Behling, H., Pillar, V., and Bauermann, S. Chiliad. (2004). Late Quaternary Araucaria forest, grassland (Campos), fire and climate dynamics, inferred from a high-resolution pollen record of Cambará practise Sul in southern Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 203, 277–297. doi: ten.1016/S0031-0182(03)00687-4
CrossRef Full Text
Boldrini, I. I. (2006). Biodiversidade dos Campos Sulinos. i Simpósio de Forrageiras e Produção Animal – Ênfase: Importβncia e Potencial Produtivo da Pastagem Nativa, Vol. one. UFRGS, Departamento de Plantas Forrageiras eastward Agrometeorologia, 11–24.
Boldrini, I. I., and Longhi-Wagner, H. K. (2011). Poaceae no rio grande practise sul: diversidade, importβncia na fitofisionomia e conservação. Ciência Ambiente 42, 71–92.
Chaturvedi, G., and Datta, K. (2001). Pollen morphology in Saccharurn L. (Poaceae) - wild and cultivated sugar cane species. Feddes Repertorium 112, 387–390. doi: 10.1002/fedr.4921120509
CrossRef Full Text | Google Scholar
Chaturvedi, M., Datta, K., and Nair, P. Yard. Grand. (1998). Pollen morphology of Oryza (Poaceae). Grana 37, 79–86. doi: x.1080/00173139809362647
CrossRef Full Text | Google Scholar
Chaturvedi, M., Yunus, D., and Datta, K. (1994). Pollen morphology of sorghum moench-sections european union-sorghum and para-sorghum. Grana 33, 117–123. doi: x.1080/00173139409428987
CrossRef Full Text | Google Scholar
Côrrea, A. M. S., Guimarães, One thousand. I. T. Thou., Cruz-Barros, M. A. V., and Begale, F. F. (2005). Flora polínica da reserva do parque estadual das fontes do ipiranga (São Paulo, Brasil). Hoehnea 32, 269–282. doi: 10.1590/S2236-89062011000100009
CrossRef Total Text
Datta, K., and Chaturvedi, M. (2004). Pollen morphology of Basmati cultivars (Oryzasativa race Indica) – exine surface ultrastructure. Grana 43, 89–93. doi: 10.1080/00173130310017391
CrossRef Full Text | Google Scholar
Dórea, G. C. (2011). Morfologia polínica, Fenologia Reprodutiva e Biologia Floral de Espécies Florestais de Poaceae. Ph.D. thesis, Universidade Estadual de Feira de Santana.
Eva, H. D., Miranda, East. Due east., Di Bella, C. M., Gond, 5., Huber, O., Sgrenzaroli, S., et al. (2002). A Vegetation Map of South America. Luxembourg: Join Research Centre. Office for Official Publications of the European Communities.
Google Scholar
Erdtman, Grand. (1952). Pollen Morphology and Plant Taxonomy. Angiosperms. Stockholm: Almkvist & Wiksell.
Google Scholar
Filgueiras, T. Due south., Brochado, A. L., Nogueira, P. E., and Guala, I. I. 1000. F. (1994). Caminhamento – um método expedito para levantamentos florísticos quantitativos. Cadernos Geociê 12, 39–44.
Grass Phylogeny Working Grouping(GPWG). (2001). Phylogeny and subfamilial classification of the grasses (Poaceae). Ann. Missouri Bot. Garden 88, 373–457. doi: x.2307/3298585
CrossRef Full Text
Hasenack, H., Weber, Eastward., Boldrini, I. I., and Trevisan, R. (2010). Mapa de Sistemas Ecológicos da Ecorregião das Savanas Uruguaias em escala ane:500.000 ou superior e Relatório Técnico descrevendo insumos e metodologia de elaboração do mapa de Sistemas Ecológicos. Relatório Técnico, The Nature Conservancy.
Heusser, C. J. (1971). Pollen and Spores of Republic of chile. Tucson: The university of Arizona Printing.
Google Scholar
Jan, F., Schüler, 50., and Behling, H. (2014). Trends of pollen grain size variation in C3 and C4 Poaceae species using pollen morphology for time to come assessment of grassland ecosystem dynamics. Grana 53, one–17. doi: 10.1080/00173134.2014.966754
CrossRef Full Text | Google Scholar
Joly, C., Barillé, 50., Barreau, Thou., Mancheron, A., and Visset, Fifty. (2007).Grain and annulus diameter equally criteria for distinguishing pollen grains of cereals from wild grasses.Rev. Palaeobot. Palynol. 146, 221–233. doi: 10.1016/j.revpalbo.2007.04.003
CrossRef Full Text
Katsiotis, A., and Forsberg, R. A. (1995). Pollen grain size in 4 ploidy levels of genus Avena. Euphytica 83, 103–108. doi: ten.1007/BF01678036
CrossRef Total Text | Google Scholar
Köhler, Eastward., and Lange, E. (1979). A contribution to distinguishing cereal from wild grass pollen grains past LM and SEM. Grana 18, 133–140. doi: 10.1080/00173137909424973
CrossRef Total Text | Google Scholar
Linder, H. P., and Ferguson, I. K. (1985). On the pollen morphology and phylogeny of the Restionales and Poales. Grana 24, 65–76. doi: x.1080/00173138509429917
CrossRef Full Text | Google Scholar
Liu, Q., Zhao, N., and Hao, 1000. (2004). Pollen morphology of the Chloridoideae (Gramineae). Grana 43, 238–248. doi: x.1080/00173130410000776
CrossRef Full Text | Google Scholar
Macedo, R. B., Cancelli, R. R., Bauermann, S. One thousand., Neves, P. C. P., and Bordignon, S. A. L. (2007). Palinologia de níveis do Holoceno da Planície Costeira practice Rio Grande do Sul (localidade de Passinhos), Brasil. Rev. Gaea Unisinos 3, 68–74. Bachelor online at: http://revistas.unisinos.br/index.php/gaea/article/view/5867/3053
Mander, L., Baker, Southward. J., Belcher, C. One thousand., Haselhorst, D. S., Rodriguez, J., Thorn, J. L., et al. (2014). Accuracy and consistency of grass pollen identification by human analysts using electron micrographs of surface ornamentation. Applications in Plant Sciences two, one–11. doi: 10.3732/apps.1400031
PubMed Abstract | CrossRef Total Text | Google Scholar
Mander, L., Li, Grand., Mio, W., Fowlkes, C. C., and Punyasena, S. W. (2013). Classification of grass pollen through the quantitative analysis of surface ornamentation and texture. Proc. Biol. Sci. 280, 1–7. doi: x.1098/rspb.2013.1905
PubMed Abstract | CrossRef Full Text | Google Scholar
Mander, L., and Punyasena, Due south. W. (2016). Grass pollen surface ornamentation: a review of morphotypes and taxonomic utility. J. Micropalaeontol. 35, 121–124. doi: x.1144/jmpaleo2015-025
CrossRef Full Text | Google Scholar
Markgraf, V., and D'Antoni, H. (1978). Pollen flora of Argentina. Tucson: University of Arizona Press.
Google Scholar
Medeanic, S., Cordazzo, C. V., and Lima, L.Thousand. (2008). Diversidade Polínica de Plantas em Dunas no Extremo Sul do Brasil. Porto Alegre: Gravel.
Melhem, T. Southward., Cruz-Barros, M. A. Five., Corrêa, Yard. A. South., Makino-Watanabe, H., Silvestre-Capelato, M. S. F., and Esteves, 5. 1000. L. (2003). Variabilidade polínica em plantas de Campos do Jordão (São Paulo, Brasil). Bol. Inst. Bot. 16, 16–104.
Google Scholar
Morgado, L. North., Gonçalves-Esteves, 5., Resendes, R., and Ventura, M. A. M. (2015). Pollen morphology of Poaceae (Poales) in the Azores, Portugal. Grana 54, 282–293. doi: ten.1080/00173134.2015.1096301
CrossRef Total Text | Google Scholar
Nakamura, A. T., Longhi-Wagner, H. M., and Scatena, V.L. (2010). Anther and pollen development in some species of Poaceae (Poales). Br. J. Biol. 70, 351–360. doi: 10.1590/S1519-69842010005000005
PubMed Abstract | CrossRef Full Text | Google Scholar
Nazir, A., Khan, Grand. A., Abbasi, A. M., and Zahidullah (2013). Palynological studies in Tribe Aveneae (Poaceae) from Potohar of Pakistan. Int. J. Sci. ten, 120–125.
Needham, I., Vorontsova, M. S., Banks, H., and Rudall, P. J. (2015). Pollen of Malagasy grasses as a potential tool for interpreting grassland palaeohistory. Grana 54, 247–262. doi: 10.1080/00173134.2015.1057220
CrossRef Full Text | Google Scholar
Perveen, A. (2006). A contribution to the pollen morphology of family gramineae. World Appl. Sci. J. i, lx–65.
Google Scholar
Punt, Westward., Hoen, P. P., Blackmore, S., Nilsson, S., and Le Thomas, A. (2007). Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 143, 1–81. doi: 10.1016/j.revpalbo.2006.06.008
CrossRef Total Text | Google Scholar
Radaeski, J. North., and Bauermann, Due south. G. (2016). Avaliação da produção polínica de Bromus catharticus Vahl e Guadua trinii(Nees) Nees ex Rupr. (Poaceae) para a interpretação de dados fósseis. Biotemas 29, 9–18.
Radaeski, J. N., Evaldt, A. C. P., and Bauermann, S. 1000. (2014a). Grãos de pólen de espécies ocorrentes na Unidade de Conservação Parque Estadual do Espinilho, Barra do Quarai, Rio Grande do Sul, Brasil. Pesqui. Bot. 65, 305–331. Available online at: http://www.anchietano.unisinos.br/publicacoes/botanica/botanica65/BOTANICA%2065.pdf
Radaeski, J. Northward., Evaldt, A. C. P., Bauermann, S. G., and Lima, G. L. (2014b). Diversidade de grãos de pólen east esporos dos Campos practise sul do Brasil: descrições morfológicas due east implicações paleoecológicas. Iheringia Série Bot. 69, 107–132. Available online at: http://www.fzb.rs.gov.br/upload/20140805153253ih69_1_p107_132.pdf
Roubik, D. W., and Moreno, J. E. (1991). Pollen and Spores of Barro Colorado Island. St. Louis, MO: Missouri Botanical Garden.
Google Scholar
Salgado-Labouriau, M. Fifty. (1973). Contribuição à Palinologia dos Cerrados. Rio de janeiro: Academia Brasileira de Ciências, 291.
Salgado-Labouriau, M. 50. (2007). Critérios e Técnicas Para o Quaternário. São Paulo: Editora Blücher.
Salgado-Labouriau, M. L., and Rinaldi, One thousand. (1990). Palynology of gramineae of the venezuelan mountains. Grana 29, 119–128. doi: 10.1080/00173139009427742
CrossRef Full Text | Google Scholar
Schüler, L., and Behling, H. (2011a). Characteristics of Poaceae pollen grains as a tool to assess palaeoecological grassland dynamics in South America. Veget. Hist. Archaeobot. twenty, 97–108. doi: 10.1007/s00334-010-0264-0
CrossRef Full Text | Google Scholar
Schüler, 50., and Behling, H. (2011b). Poaceae pollen grain size as a tool to distinguish past grasslands in South America: a new methodological arroyo. Veget. Hist. Archaeobot. xx, 83–96. doi: 10.1007/s00334-010-0265-z
CrossRef Full Text | Google Scholar
Skvarla, J. J., Rowley, J. R., Hollowell, V. C., and Chissoe, W. F. (2003). Annulus-Pore relationship in Gramineae (Poaceae) pollen: the pore margin of Pariana. Am. J. Bot. ninety, 924–930. doi: 10.3732/ajb.90.6.924
PubMed Abstract | CrossRef Total Text | Google Scholar
Tedesco, South. B., Battistin, A., and Valls, J. F. 1000. (1999). Diβmetro dos grãos de pólen eastward tamanho dos estômatos em acessos diplóides e tetraplóides de Hemarthria altissima (Poiret) Stapf & Hubbard (Gramineae). Santa Maria. Ciência Rural 29, 273–276. doi: ten.1590/S0103-84781999000200014
CrossRef Total Text | Google Scholar
Wilberger, T. P., Stranz, A., Paz, C., Boeni, B., Cancelli, R. R., Bauermann, S. B., et al. (2004). Flora do Setor Oriental do Planalto sul-rio-grandense. Guia de espécies vegetais.1a Edn. São Leopoldo, ALPP.
Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpls.2016.01833/full
0 Response to "Grass Pollen Surface Ornamentation a Review of Morphotypes and Taxonomic Utility"
ارسال یک نظر